
Making Medicine - The Drug Development Process
Developing a new HIV drug can take up to 15 years before it makes it into your medicine cabinet. It often involves reviewing thousands of substances to find ones that are active against HIV. According to the FDA, only 1 in 1,000 compounds makes it from the lab to clinical trials in humans. From there, only 1 in 5 approved.
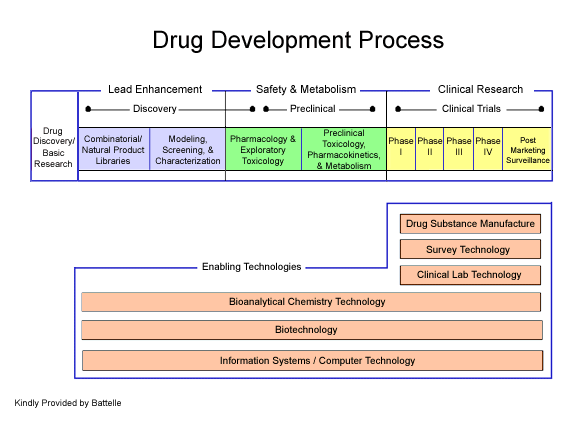
Stages of Drug Development
Pre-Clinical
- Most HIV drug candidates are identified by testing existing drugs for anti-HIV activity (screening). A newer method is rational drug design, where scientists "build" drug molecules to fight HIV in specific ways.
- When a promising drug is identified, it goes through pre-clinical testing – with test-tube and animal studies. These studies show if the drug works against HIV and how it works. They also make sure that it is not too toxic (poisonous).
- If results from pre-clinical testing show promise, the researchers file an Investigational New Drug (IND) application with the Food and Drug Administration (FDA). Then they can start testing the drug in humans (clinical trials).
Phases of a Clinical Trial: There are four phases of human clinical trials.
- Phase I
A drug candidate is given to people for the first time to test its SAFETY on humans. There are usually about 10-100 participants during this phase, and the trial usually takes less than a year.
- Phase II
This phase determines how well the drug WORKS AGAINST HIV and continues to study SAFETY and SIDE EFFECTS. Several hundred people participate in Phase II, and the study can last 1-2 years.
- Phase III
This phase studies the EFFECTIVENESS of the drug and continues to look at TOXICITY and possible LONG-TERM SIDE EFFECTS. This phase involves the largest number of participants (typically hundreds or thousands), and the study often lasts for a year or more.
Once Phase III trials are successfully finished, researchers may submit a New Drug Application (NDA) to the FDA. The FDA will review the results from these studies to determine whether a drug should be approved for use to treat specific medical conditions.
- Phase IV
Known as “post-marketing studies,” Phase IV occurs after a drug hits the market and people have been using it. It monitors long-term effectiveness, safety under "real world" conditions, and are especially valuable for detecting long-term and uncommon side effects that do not show up in earlier trials. This phase also compares the drug to similar drugs on the market.
Links to:
History of HIV Drug Therapy
Classes of HIV Drugs and Treatments
ARA Scientific Domains

